Summary of all activities of the Metallic Construction Materials group is here.
Hydrogen embrittlement poses a problem in various industrial sectors, including the energy, aerospace, automotive, and marine industries. Hydrogen can enter a material when in contact with a corrosive environment or during exposure to hydrogen gas. When hydrogen interacts with metal, especially under increased load, it can lead to a loss of mechanical properties, particularly ductility. To assess the susceptibility of a material to hydrogen embrittlement, it is crucial to determine the critical concentration of hydrogen that causes damage. This parameter can be established by charging samples with hydrogen to a specific level and subsequently conducting mechanical tests.
For a deeper analysis, the permeation test is an effective tool. It allows to determine the rate of hydrogen flow through the material and the capture of hydrogen in hydrogen traps. When the density of these traps is adjusted, for example, through changes in heat treatment, hydrogen retention can be influenced, thereby reducing the risk of hydrogen embrittlement. The binding energy of individual traps and the hydrogen content in the material can be determined using thermal desorption analysis.
When studying hydrogen absorption during atmospheric corrosion, a scanning Kelvin probe can be used, which enables visualization of the hydrogen flow through a sample with a very high sensitivity. The ratios of cathodic reactions during the corrosion process, oxygen reduction, and hydrogen evolution, can be determined using respirometry.
Hydrogen chargingHydrogen charging serves to partially or completely saturate sample with hydrogen for further analyses and tests, such as the analysis of trapped hydrogen, desorption kinetics and mechanical behaviour. We use three methods of hydrogenation: Electrochemical chargingElectrochemical charging is used for the hydrogenation of samples under controlled conditions. On the basis of years of experience, we select the most suitable saturation parameters, including electrolyte composition, current density, potential, and duration of saturation, to achieve the desired level of the hydrogen concentration. This method allows for precise control of charging conditions, is highly reproducible, and is faster than the other charging methods. Corrosion chargingHydrogen is absorbed during the corrosion process. This type of saturation allows for the simulation of hydrogen entry into the material in a real environment. Exposures can be performed in any accelerated corrosion test, or under immersion in an electrolyte solution, or at constant humidity after manual application of a corrosion activator. After removal of corrosion products, the amount of hydrogen adsorbed during the exposure can be measured. Corrosion charging can also be done before performing tests at slow strain rate deformation to evaluate the influence of corrosion-induced hydrogen on mechanical properties. Charging in pressurized hydrogenAutoclave exposure can simulate real environmental conditions in high-pressure hydrogen, such as in the gas industry. Exposure can occur in dry gas, in combination with immersion, or steam exposure. For exposures up to 100 bar and 150 °C, pure hydrogen or a mixture of hydrogen with other gases can be used. The exposure time ranges from several days to several months, depending on the simulated conditions. Hydrogen desorption after the exposure can be eliminated by storing samples in liquid nitrogen until an analysis is performed. |
|
Slow strain rate testTesting at a slow strain rate provides time for hydrogen to diffuse into critical regions and eventually affect the mechanical properties. By comparing the stress-strain curves for samples containing hydrogen with those of reference samples without hydrogen, changes in ductility, strength, and cross-sectional area can be evaluated. The determination of the hydrogen embrittlement index is an important criterion when assessing the resistance of materials to hydrogen.
The first step involves designing and preparing flat or cylindrical samples with geometries that meet the requirements of ISO 6892. Coated samples can also be prepared if the effect of the coating on the hydrogen entry and behaviour is of interest. Samples may be precharged with hydrogen electrochemically, during exposure to a corrosive environment, or in hydrogen gas. To maintain a constant high hydrogen concentration in the material, electrochemical or corrosion charging can be continued during the tensile test, which is especially important for steels with high hydrogen diffusion and desorption rates.
The tensile test is performed on a tensile machine at a strain rate of typically 4∙10-7 s-1. Both hydrogen-charged and hydrogen-free reference samples are tested for comparison. The test outputs are stress-strain curves that provide information on the fracture behaviour. In addition to analysis of the changes in ductility and strength caused by the presence of hydrogen, the change in the cross-sectional area and fractography analysis are also carried out.
The hydrogen embrittlement index is calculated on the basis of changes in ductility, strength, or cross-sectional area after rupture. The percentage change in one of these properties between hydrogen-charged and hydrogen-free samples is evaluated.
After a sample fractures, fractographic analysis is conducted using a scanning electron microscope. This analysis helps characterize the fracture surfaces and provides further insights into the effect of hydrogen on the material. A change in fracture type from ductile to brittle is typical of hydrogen-induced damage. However, when the hydrogen concentration in the material is low, a detailed analysis of less significant changes in the fracture surface is required. |
|
Computed microtomographyThe Diondo d2 microtomograph offers a wide range of possibilities for material studies, including the analysis of damage mechanisms caused by hydrogen. This nondestructive method enables a detailed 3D reconstruction of sample structures. The principle of the method lies in measuring the intensity of the X-ray radiation after passing through a sample. The material interaction includes radiation absorption and scattering. The resulting model is constructed from projections obtained using advanced mathematical algorithms.
The micro-tomograph is an effective tool for understating into crack propagation. One possibility is interrupting a slow strain rate test and performing measurements, comparing a hydrogen-charged sample with a hydrogen-free reference sample. Another option is to scan the sample while applying a constant load using a loading stage. Such a tensile test can provide valuable real-time insights into the fracture behaviour in presence of hydrogen.
Thanks to the device's high resolution of up to 2 µm, it is possible to obtain detailed images of microcracks in the entire volume of the material. Measurements of crack length, width, and propagation direction can be made. |
 |
| Loading stage for applying stress during scanning |
Thermal desorption analysis
This method is based on heating or fusion of a sample in inert carrier gas. The amount of hydrogen present in the metal is determined by measuring the change in thermal conductivity of the carrier gas caused by hydrogen desorbed from the sample. Before the analysis, metal samples are charged using one of the procedures described above and stored in liquid nitrogen to prevent hydrogen desorption prior to the measurement. The total hydrogen content can be determined in an impulse furnace by melting the sample in an inert gas. Diffusible hydrogen content can be analysed by heating the sample in an external infrared furnace to a desired temperature. Released hydrogen is carried by the inert gas to the detection system, where a thermal conductivity detector (TCD) determines the absolute hydrogen content with an accuracy of 0.01 ppm. The typical output is a desorption curve, from which the amount of absorbed hydrogen can be determined through integration. This curve can also reveal additional hydrogen characteristics of the material, such as the type and density of hydrogen traps in the metal microstructure.
It is possible to analyse metals with high melting points, such as Ti, Ta, Zr, Nb, and their alloys, as well as all common metals and their alloys.
 |
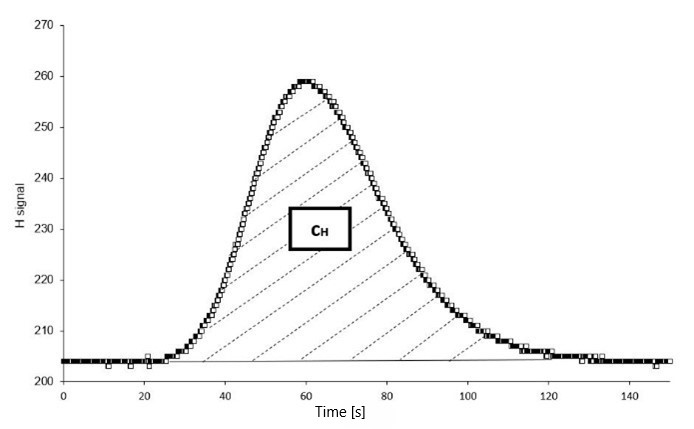 |
| Bruker G8 Galileo hydrogen analyser with external infrared furnace (left) and output of the analysis, a hydrogen desorption curve (right) | |
Electrochemical permeation test
The electrochemical permeation test can be used to determine the diffusion coefficient of hydrogen and the density of hydrogen traps in the studied material. This information is crucial for assessing the risk of hydrogen embrittlement and subsequent material selection or optimization.
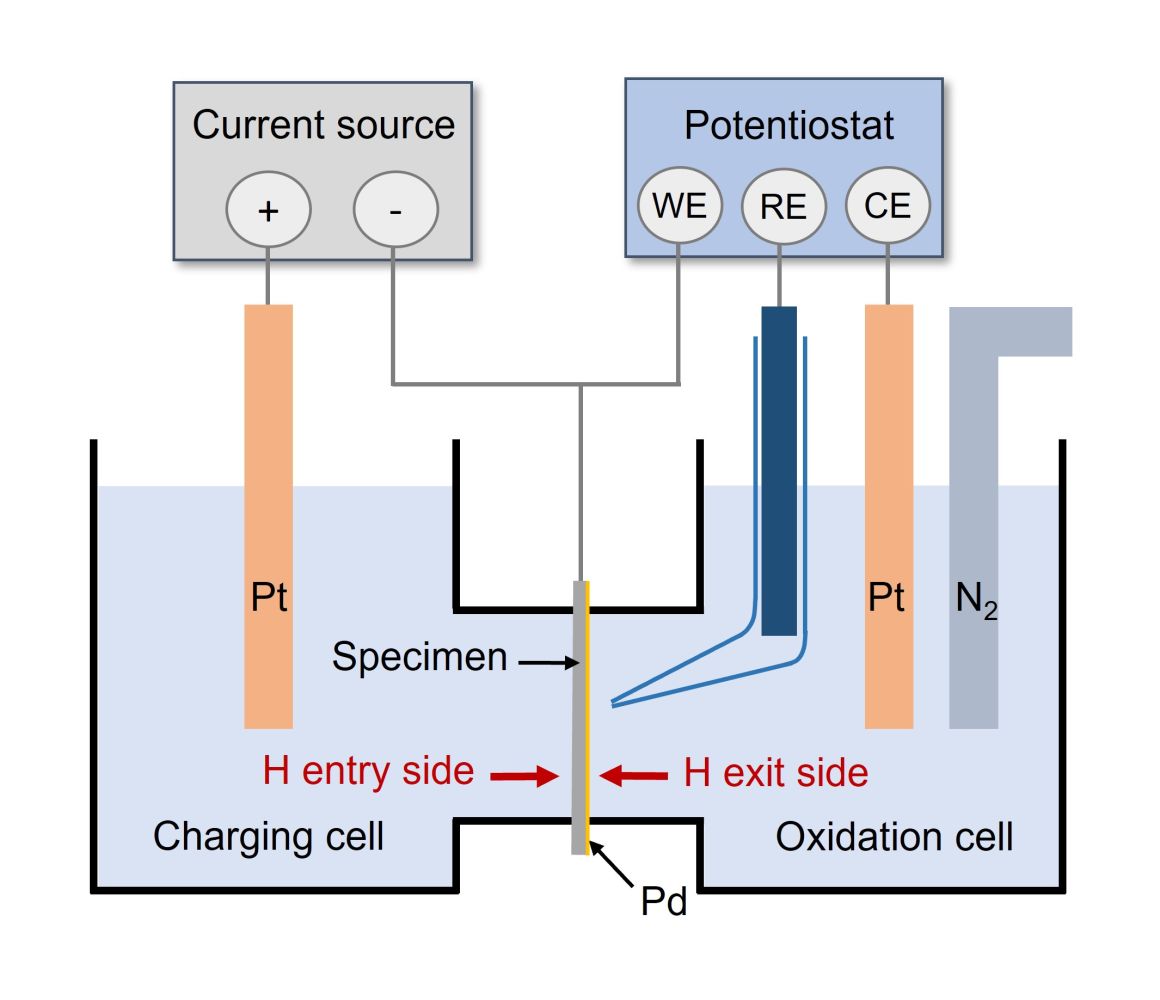
A thin, flat sample of the studied material serves as a membrane between two cells: a charging cell and a detection cell. In the classic setup, the sample is electrochemically charged, and hydrogen passing through the sample is detected on the opposite side of the membrane. In the detection cell, the sample is anodically polarized, and the current density is recorded. Diffusing atomic hydrogen is oxidized to ions, which results in an increase in the measured current. To determine the diffusion coefficient, both the standard calculation and permeation curve fitting using a numerical method can be used. Alternating the initiation and restriction of hydrogen entry on the charging side allows for monitoring of hydrogen trapping. The rate of current decay on the detection side provides information on the density of reversible hydrogen traps, which is critical in terms of hydrogen embrittlement risk.
The permeation test can be modified so that instead of electrochemical charging, the sample is exposed to atmospheric corrosion or immersed in an electrolyte solution. This modification makes the permeation test a suitable method for monitoring hydrogen flux through a material under conditions that simulate a real corrosion environment, such as immersion in seawater.
The current on the detection side of the sample is measured using a highly sensitive potentiostat Biologic SP-200 in a three-electrode setup. The permeation test is extremely sensitive even to small hydrogen fluxes through the sample, but requires careful sample preparation. A key step is the application of a palladium layer on the detection side of the sample. This palladium layer prevents corrosion during anodic polarization, which could otherwise lead to measurement artifacts.
 |
|
Schematic of an electrochemical permeation test cell |
Scanning Kelvin probe
Scanning Kelvin probe is an effective method for hydrogen detection in metals, although it is also used for other research tasks. Kelvin probe measurements provide information on the potential distribution across the sample surface under atmospheric conditions. The probe measures a contact potential difference between two electrodes, the probe and the sample.
The principle of hydrogen detection is based on the interaction of hydrogen with the surface layer on the metal. For steel, this involves a natural oxide layer, where hydrogen alters the Fe²⁺ to Fe³⁺ ratio, leading to a decrease in the measured potential at the detection site. For other metals, palladium can be deposited on the surface, which acts as a hydrogen electrode. When hydrogen flows locally through the material, regions with a lower potential relative to the hydrogen-free reference area are visualized.
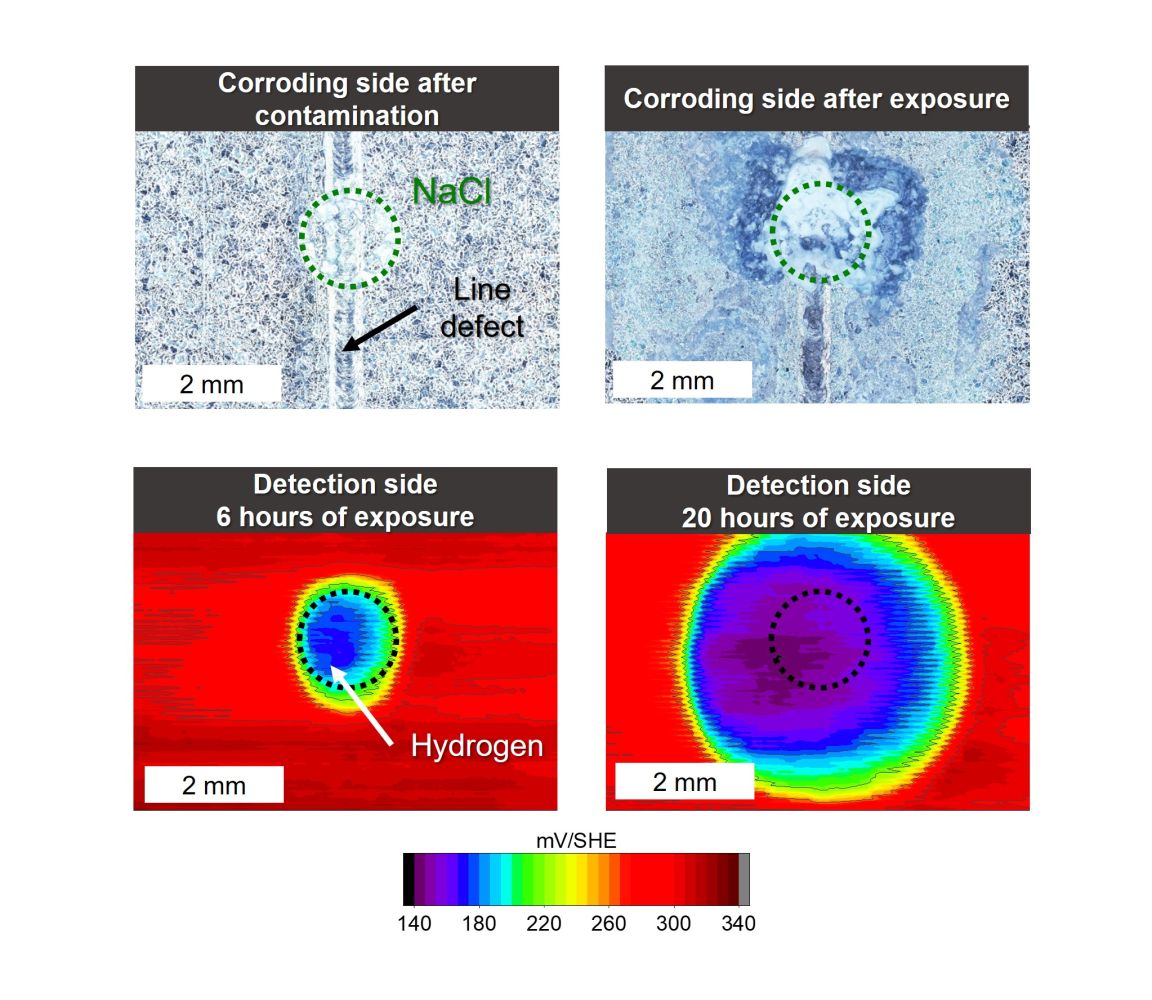
This method is particularly useful for the study of hydrogen entry under atmospheric conditions. The sample, similar to the permeation test, acts as a membrane for hydrogen. On one side, the sample corrodes, hydrogen enters the metal, diffuses through it, and is detected on the other side using the Kelvin probe. The device is equipped with a unique cell that ensures humidity control on the corroding side of the sample. A camera system regularly captures the progression of the corrosion reaction. Various exposure conditions can be set on the corroding side of the sample, such as the amount of corrosion activator, localized or uniform contamination, and relative humidity, including the alternation of dry and wet phases. The sample can be coated or bare. For coated steel with a coating defect, the effect of a galvanic cell on hydrogen entry can be studied.
 |
|
Example of hydrogen detection in a zinc-coated steel sample; Top: Corroding side of the sample with an artificial coating defect, locally contaminated with a sodium chloride solution droplet; Bottom: Potential maps measured on the opposite side of the sample, where hydrogen is detected |
The method is not limited to corrosion-induced hydrogen entry. Using the Kelvin probe, it is also possible to observe hydrogen desorption from the surface of a precharged sample.
Parallel measurements using a permeation test under the same conditions on the entry side allow for conversion of potential drop values to the amount of hydrogen absorbed into the metal. This enables a quantitative comparison of hydrogen entry efficiency under different conditions.
The main advantages of the Kelvin probe are its high sensitivity even to low hydrogen flux through the sample and its ability to monitor localized hydrogen entry. The probe has a lateral resolution of approximately 150 µm. Potential maps provide information on the hydrogen entry distribution across the sample surface, while faster potential profile measurements along a line are used for studying hydrogen desorption kinetics.
 |
 |
|
Scanning Kelvin probe (SKP) from Wicinski-Wicinski equipped with a humidity control cell and a camera system |
|
Respirometry
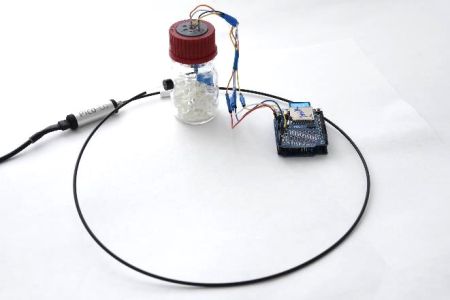
Respirometry is a technique suitable for studying the evolution of molecular hydrogen under conditions of atmospheric corrosion and corrosion in an electrolyte solution. It is accompanied by both oxygen reduction and hydrogen formation, with possible subsequent absorption into the metal. Respirometry enables in situ monitoring of the corrosion process and determination of the ratio between the ongoing cathodic reactions over time.
Changes in partial pressures of oxygen and hydrogen in a cell with corroding samples are monitored. After a corrosion activator is applied, the samples are exposed to a humid environment in a hermetically sealed cell. The cell is equipped with two sensors: an optical non-contact oxygen sensor and a temperature, pressure, and humidity sensor. These sensors detect changes in the partial pressure of oxygen and the total pressure of the system. On the basis of the differences in these parameters, it is possible to determine the pressure change caused by the hydrogen evolution. The the kinetics of the overall corrosion process as well as individual cathodic reactions can be followed. High sensitivity of the measurements enables the detection of only small changes in the reaction ratio.
After the respirometry measurements, the amount of hydrogen absorbed into the samples can be determined using thermal desorption analysis.
The respirometry method provides valuable information for understanding into and modelling corrosion processes.
 |
|
Respirometry, an experimental setup |
About us
Technopark Kralupy is a spin-off of The University of Chemistry and Technology Prague serving the Czech and international industry in the field of building chemistry and similar subjects since 2015.
Contact
Department of Metallic Construction Materials
Technopark Kralupy of the University of Chemistry and Technology Prague
Technopark Kralupy VŠCHT Praha
Náměstí G. Karse 7
278 01 Kralupy nad Vltavou
Czech Republic
kovy@technopark-kralupy.cz
Phone: +420 220 446 104, +420 723 242 413
© 2022–2025 Technopark Kralupy




