← Home | → ALCOAT in a nutshell | How do we do that | → Expected outcome | → Team | → Further information | → Contact
ALCOAT PROJECT
How do we do that
|
Recent research data indicate that aluminium-based coatings capable of providing sufficient sacrificial effect together with prevention of hydrogen uptake can be developed by applying advanced alloying, micro-alloying, and microstructure modification of aluminium. To develop aluminium-rich alloy coatings with corrosion potential more noble than that of traditional hot-dip galvanized zinc but active enough to protect steel in defects, Al will be alloyed with Fe and other metals and its microstructure modified by heat treatment. |
|
Alloying elements play a key role in the properties of Al alloys and coatings. The common aim is to design an alloy coating that would corrode uniformly and sufficiently slowly, providing in parallel high efficiency as a sacrificial anode to prevent the occurrence of red rust on steel in defects. The sacrificial efficiency must be obtained without decreasing the free corrosion potential of the coating too much in order to prevent hydrogen embrittlement of steel. Different alloying elements are known to affect the properties of Al coatings. The main expected effect is destabilisation of the Al2O3-based passive layer. Zinc, tin, and indium are well known to enter the Al2O3 lattice, increase ionic conductivity, and allow for uniform dissolution of Al. Zinc is especially efficient because it is well soluble in aluminium and thus enters the lattice of the passive layer from a solid solution over the whole surface. Tin and indium are less soluble, forming phases at the grain boundaries of the primary α-Al. The alloying elements also refine the microstructure of the alloy, improving the uniformity of the metal dissolution. Metals such as indium, gallium, and bismuth are nobler and can re-deposit on the surface near anodic particles, damage the passive layer, and keep coatings electrochemically active. |
|
Data obtained in previous research at the University of Chemistry and Technology in Prague showed that the presence of iron also has an activation effect on aluminium. This finding will be used for the development of coatings that contain very little or no heavy metal activators. Al-Fe-X(-Y) coatings, where X can be Zn and Y a microalloying element, have a strong potential to replace zinc coatings, because their electrochemical properties can be precisely tailored for different applications. |
|
Current aluminium recycling methods do not allow efficient removal of iron contamination from Al scrap. If secondary aluminium alloys contain more than 0.3 wt. % iron, the formation of Al-Fe intermetallic particles with needle morphology makes them susceptible to mechanical damage. Today, the only available process for recycling iron-containing Al scrap is dilution by pure Al. Consequently, Al scrap with a higher Fe content is unwanted and often landfilled. This project has a special objective of using iron-containing aluminium scrap, which is not suitable for the production of aluminium alloys. Technologies allowing for circular use of this unwanted scrap at industrial scales will be investigated. The higher iron content and somewhat lower ductility are not a problem for the coatings planned to be developed. On the contrary, the Fe content close to the eutectic concentration of approximately 1.8 wt.% leads to a very fine microstructure, which is advantageous for corrosion uniformity and increased sacrificial efficiency. This is not only technically advantageous but also economically attractive for future producers. This can be demonstrated by the relative price of Al scrap as a function of the iron content. If contaminated with more than 2 wt. % iron, it is sold at 50% discount. If it contains more than 5 wt. % iron, the price drops by 85 %. |
|
In general, metallic coating development is a long and extremely costly process with numerous iterations. This is because many, often contradictory, requirements need to be fulfilled, including low self-corrosion, efficient substrate corrosion protection, appearance, mechanical properties, manufacturing properties, uniformity, etc. Many series of coated sheet samples have to be produced and evaluated during development with a constant risk of failure and the necessity of going back to the beginning. |
|
In ALCOAT, a highly innovative concept of coating development is applied based on the in-silico approach. Input electrochemical, microstructure, and corrosion data for the advanced computational model will be obtained under laboratory conditions using easy-to-prepare model cast alloy samples. Experimental coatings will be employed only in the final development stage. This approach has the potential to dramatically reduce the time to market and development costs of coated products. A multiscale framework will be required to assess the effectiveness of Al-Fe-X(-Y) coatings in protecting the underlying steel and their durability over time. It will contain three components that are absolutely at the state-of-the-art: surface state, coating degradation, and galvanic prediction. It will incorporate comprehensive understandings developed during the last decade on the role of moisture layers in controlling coupling, the effect of precipitation on changing potential and charge distribution in the coupled system. There are two aspects where the proposed structure extends beyond current knowledge and is critical to delivering rapid in silico solutions in the optimum composition selection. Firstly, the combination of density functional theory (DFT), quantum mechanical/molecular mechanical (QM/MM), and non-equilibrium Greens function (NEGF) simulations when combined with high-throughput calculation methods and parallel high performance will deliver for the first time rapid evaluation of electrode potentials and Tafel slopes of the Al-Fe-X(-Y) systems at the highest accuracy and reliability. This will be possible because of the nano-level incorporation of polarisation features. Then, the multiscale linking of these nano-features across all scales up to the component and environmental scale (from 10-10 metres to 100 meter) will provide a detailed understanding of the impact of the nano-properties on component in-service performance. This width-of-scale linkage based on explicit models, not statistical methods, will be an international first, allowing for rapid and efficient selection of best compositions for the coating development in the second stage of ALCOAT. |
|
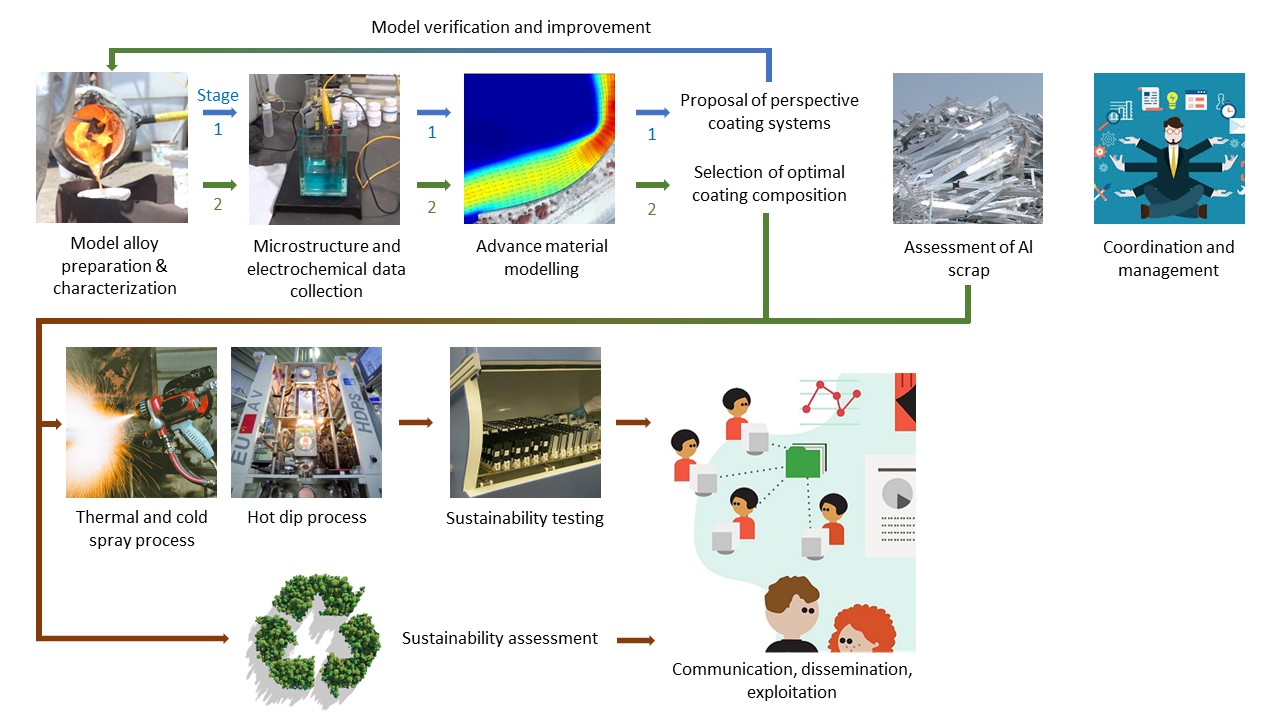
The ALCOAT approach can be summarised as follows.
|
← Home | → ALCOAT in a nutshell | How do we do that | → Expected outcome | → Team | → Further information | → Contact

 Current aluminium coatings
Current aluminium coatings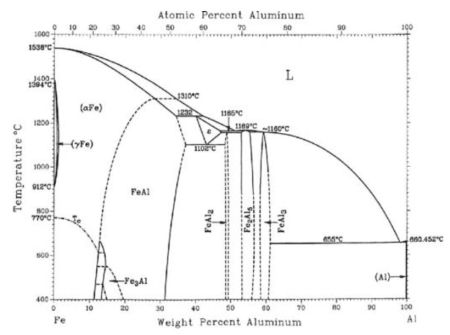 Aluminium alloying
Aluminium alloying Iron as an alloying element
Iron as an alloying element Iron-polluted aluminium scrap
Iron-polluted aluminium scrap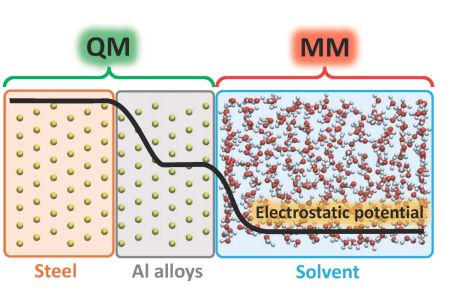 Coating development process
Coating development process Modelling for coating development
Modelling for coating development